Mapping microbial frontiers: Gut geography unlocks new avenues for disease therapy
FAYETTEVILLE, GA, UNITED STATES, July 18, 2025 /EINPresswire.com/ -- The gut is home to trillions of microbes that play a pivotal role in digestion, metabolism, and immune function. Traditionally, microbiome research has focused on the colon, but emerging studies show that microbial populations in other regions of the gastrointestinal (GI) tract, such as the small intestine, are equally crucial in disease development. Recent implications suggest that microbial displacement—when bacteria migrate from their native regions to areas where they don’t belong—may contribute to metabolic and gastrointestinal diseases. This perspective proposes that by understanding and targeting the specific microbial communities along the GI tract, we may unlock new therapeutic strategies to treat metabolic and gastrointestinal disorders like obesity, type 2 diabetes, liver disease, inflammatory bowel disease (IBD), and others.
Gut microbiome research has primarily concentrated on the colon and fecal samples, but a recent perspective reveals a much more complex microbial geography across the entire GI tract. Each segment of the gut, from the stomach to the colon, hosts unique microbial populations shaped by its distinct physiological environment. In particular, the small intestine’s role in nutrient absorption and immune regulation has been undervalued. Disruption of this delicate microbial balance, caused by factors like diet, illness, or aging, can lead to a cascade of metabolic dysfunctions. Due to these emerging insights, there is an urgent need to study the regional specificity of gut microbiota to develop more precise interventions for gut-related diseases.
A recent perspective (DOI: 10.1093/procel/pwae058) published on October 29, 2024 in Protein & Cell by researchers from Shanghai Sixth People’s Hospital and the University of Hong Kong, shifts focus to the often-overlooked microbial communities of the small intestine. This piece of perspective explores how the movement of microbes between different regions of the gastrointestinal tract can disrupt metabolic homeostasis, potentially contributing to diseases such as obesity and type 2 diabetes. This perspective challenges traditional views and paves the way for new microbiome-based therapeutic strategies that address the root causes of metabolic and gastrointestinal disorders.
In their paper, the authors examined how the microbial composition of different GI regions affects host metabolism. The small intestine, despite its lower microbial biomass compared to the colon, plays a crucial role in digestion and nutrient absorption. The authors highlight that when microbes migrate to non-native regions, like the duodenum or jejunum, they can disrupt bile acid processing, a critical pathway for maintaining metabolic health. This disruption contributes to phenomena like small intestinal bacterial overgrowth (SIBO), which is linked to conditions like metabolic syndrome and insulin resistance.
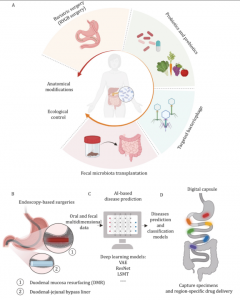
The perspective also emphasizes the complex interplay between bile acids and microbes: bile acids regulate microbial growth and gut immunity, while gut microbes transform bile acids. Microbial dislocation disrupts bile acid metabolism, leading to dysregulation of glucose and lipid metabolism through impaired signaling pathways (e.g., FXR and TGR5). In the upper small intestine, bile acids inhibit bacterial overgrowth, while in the lower gut, microbial transformations of bile acids support symbiont proliferation. When microbial dislocation disturbs this balance, it affects critical signaling pathways involved in glucose and lipid metabolism. The authors propose that targeted therapies, such as fecal microbiota transplantation (FMT) or its optimized alternative—whole-intestinal microbiota transplantation (WIMT), personalized probiotic/prebiotic interventions, bacteriophage therapy, or endoscopic/surgical interventions, could restore microbial balance and alleviate metabolic diseases.
This perspective represents a paradigm shift in how we view the gut microbiome, said Dr. Wei Jia, corresponding author of the paper. While the colon has traditionally been the focus of microbiome research, this perspective shows that disturbances in the microbial communities of the small intestine are just as influential in the onset of metabolic diseases. By understanding these regional microbial ecosystems, we open new doors for more personalized and effective treatments. And the first author Junliang Kuang adds: By targeting the microbial distribution along the entire GI tract, we can potentially treat the root causes of diseases like diabetes and obesity, rather than just managing symptoms.
This perspective has significant implications for the treatment of metabolic and gastrointestinal diseases. Targeting specific microbial populations throughout the GI tract (including those in the small intestine) could offer a more effective approach than broad-spectrum treatments currently available. Therapies like duodenal mucosal resurfacing and bariatric surgery, which alter the GI tract’s anatomy, show promise in restoring microbial balance and improving metabolic health. Furthermore, AI-driven prediction models using multidimensional data (including oral and fecal microbiota) could identify at-risk individuals before disease onset, enabling earlier intervention. As research progresses, personalized microbiome-based therapies tailored to individual gut microbial profiles may greatly advance the treatment of metabolic and gastrointestinal disorders.
References
DOI
10.1093/procel/pwae058
Original Source URL
https://doi.org/10.1093/procel/pwae058
Funding Information
This work was supported by the National Science and Technology Major Project of China (2023ZD0507602), the National Natural Science Foundation of China (82270917, 82470853).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.